Solid-Liquid Equilibria (VLE): Property Name & Phase Formats
The example shows VLE data of various types for (ethylbenzene + p-xylene). Property Names are written out explicitly for Phase boundary pressure and Mole fraction (i.e., composition).
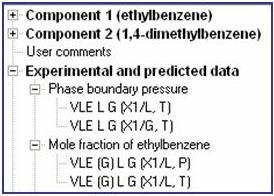
VLE (in the figure) indicates the general type of phase equilibrium data displayed. Property Phases are listed below the property name and are interpreted as follows:
Phase Boundary Pressure
- L G (X1/L, T): The pressure along the LIQUID-GAS saturation line as a function of the mole fraction of component 1 (ethylbenzene) in the liquid phase L and temperature T
- Commonly referred to as Bubble Point or P-T-x data.
- L G (X1/G, T): The pressure along the LIQUID-GAS saturation line as a function of the mole fraction of component 1 (ethylbenzene) in the gas phase G and temperature T.
- Commonly referred to as Dew Point or P-T-y data.
Mole fraction of ethylbenzene
- (G) L G (X1/L, P): The mole fraction of ethylbenzene in the gas phase (G) along the LIQUID-GAS saturation line L G as a function of the mole fraction of component 1 (ethylbenzene) in the liquid phase L and pressure P.
- Commonly referred to as P-x-y data.
- (G) L G (X1/L, T): The mole fraction of ethylbenzene in the gas phase (G) along the LIQUID-GAS saturation line L G as a function of the mole fraction of component 1 (ethylbenzene) in the liquid phase L and temperature T.
- Commonly referred to as T-x-y data.
See also Property Name & Phase Formats for Mixtures and Pure Components.