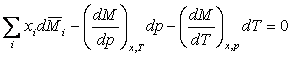
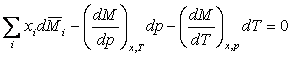
where M is a molar thermodynamic property, Mi bar is a partial molar property, and T, p, and x are temperature, pressure, and liquid composition, respectively. The summation is over the i components in the chemical system. If the property M is the excess Gibbs energy divided by the product of the gas constant R and T, then
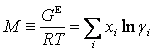
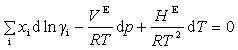
where g is an activity coefficient, VE is the excess volume and HE is the excess enthalpy. The above 3 equations are the foundation for the consistency tests in TDE. The four consistency tests considered determine the quality of a VLE data set on a pass-fail basis. Although these tests can be useful for rejection of VLE data sets with inconsistencies, it is difficult to decide whether to accept or reject a data set, when test results conflict. For such cases, quantitative test results can be very helpful. In TDE, quality factors Ftest,i for each test are evaluated as values ranging from 0.025 to 0.25, resulting in the sum of the factors having values ranging from 0.1 to 1.
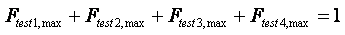 , if all tests are passed.
, if all tests are passed.
The quality factors for each test are combined alegebraically to produce the overall TDE Quality Factor QVLE. A check of consistency is also made between VLE data-set "endpoints" (i.e., mole fraction composition approaching 0 or 1) and pure-component vapor pressures. This is termed the Pure Component Consistency Test (DETAILS), and a 5th quality factor Fpure is derived.
The TDE Quality Factor QVLE is used as a weighting factor in model-fitting procedures in TDE and is calculated based on the results of all tests as follows:
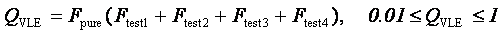 , with the limits indicated.
, with the limits indicated.
The numerical value of the TDE Quality Factor QVLE is listed on the VLE Data Sets and Consistency Tests Form following application of the consistency tests.