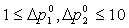VLE Thermodynamic Consistency: Pure Component Consistency Test background
Pure Component Consistency Test. In addition to the requirements related to the Gibbs-Duhem equation described, consistency is checked between the "end-points" of the VLE curve (i.e., mole fraction composition approaching 0 or 1) and the pure-component vapor pressures, as desctibed by Kang et al37. The check is made for both the liquid-phase compositions x and gas-phase compositions y.
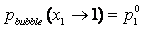 and
and 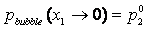
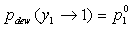 and
and 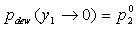
If the data set type is T-p-x-y, these equations are equivalent. For T-p-x data, the first is used, and for T-p-y data, the second equation is used. If no experimental values are reported at x1 = 0 or x1 = 1 in the VLE set, the values of Δ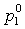 and Δ
and Δ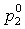 can be calculated with extrapolated vapor pressures derived with the 3-parameter NRTL model. The vapor pressures and three NRTL parameters (five parameters in total) are fitted to experimental VLE values with the following conditions:
can be calculated with extrapolated vapor pressures derived with the 3-parameter NRTL model. The vapor pressures and three NRTL parameters (five parameters in total) are fitted to experimental VLE values with the following conditions:
- the data set is isothermal,
- the minimum number of experimental values is 8,
- there is, at least, one experimental value in the composition range x1 < 0.2 to allow estimation of the vapor pressure for component 2,
- there is a minimum of one value in the composition range from x1 > 0.8 to allow estimation of the vapor pressure for component 1.
If the data set is isobaric or any of the four conditions are not met, the values of Δ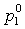 and Δ
and Δ![]() are replaced by the average deviation in bubble or dew pressure calculated with the 3-parameter NRTL model fit based on pure component vapor pressures.
are replaced by the average deviation in bubble or dew pressure calculated with the 3-parameter NRTL model fit based on pure component vapor pressures.
Unlike the four consistency tests based on the Gibbs-Duhem equation, this test can be performed for T-p-x or T-p-y data sets. The quality factor associated with the Pure Component Consistency Test can be calculated with either bubble or dew pressures, and is defined as follows:
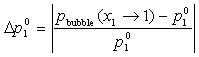 or equivalently
or equivalently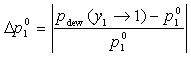 , with a lower limit of 1 and upper limit of 10.
, with a lower limit of 1 and upper limit of 10.
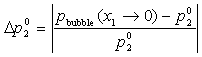 or equivalently
or equivalently 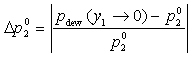 , with a lower limit of 1 and upper limit of 10.
, with a lower limit of 1 and upper limit of 10.
If the extrapolated vapor pressure agrees within 1% of pure po for both components, the factor Fpure is 1. If the vapor-pressure inconsistency is larger, the factor is calculated with the following expression:
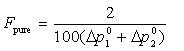 , with limits:
, with limits: