Binary Equation of State (EOS): Peng-Robinson (Original, Non-translated)
The equation is: p = RT/(V - b) - a/(V2 + 2Vb - b2)where p is the pressure, V is the molar volume, R is the gas constant, T is the temperature, a and b are mixture-specific functions of T and composition with the mixing rules
a = SSxixjaij, where the summations are over i and j, respectively.
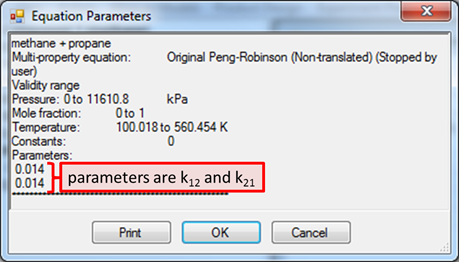
and aij = (1 - kij) (aiaj)0.5; plus, the interaction parameters are symmetrical i.e., kij = kji)
b = Sxibi, where the summation is over i.
The quantities ai and bi are the a and b parameters for each component, as calculated here;
ai= 0.45724(RTc)2 /pc,i(1 + ki(1 - (T/Tc,i)0.5))2
ki = -0.26992×wi2 + 1.54226×wi + 0.37464
bi = 0.0778×RTc/pc,i
Tc,i, pc,i, and wi are the critical temperature (in K), the critical pressure (in kPa), and the acentric factor (See reference TDE 2 [2007], Eqs. 5-15).
Interaction parameters kij can be fitted to the available mixture data using the Model Fitting Control Center, as is done for the fitting of activity-coefficient models for binary mixtures.