PPDS 2: Cpo(Ideal Gas)
Cpo/R = Clow + (C ¥ - Clow) ×y2 ×{1 + (y - 1) å(ai × yi)} ; where the summation is from i = 0 to 4.
Clow and C ¥ are equation constants, and y = T / (T + TS), where TS is a constant.
Evaluation Results:
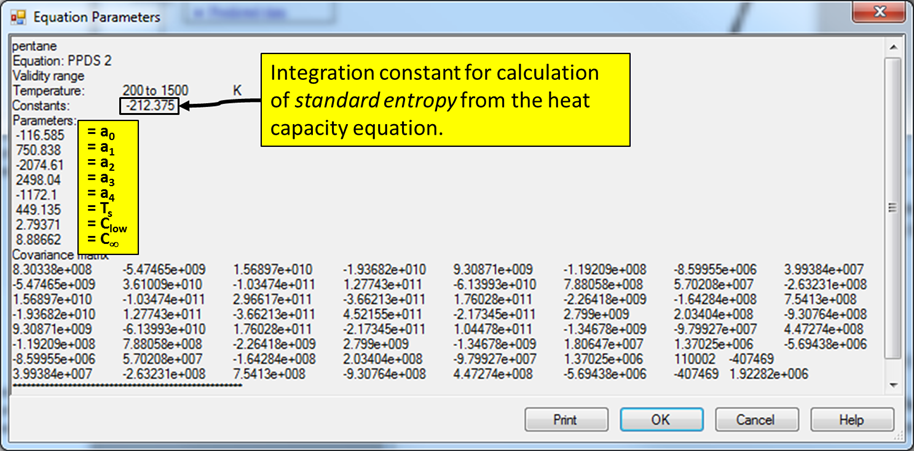
The example is for fitted heat capacities in the ideal-gas state at p = 100 kPa for pentane.