Wilhoit Equation: Ideal-gas Heat Capacity Cpo and derived properties
Cpo / R = a0 + (a1/T 2) × exp(-a2 /T ) + a3×y2 + {a4 - a5 /(T - a7)2}×y 8where R is the gas constant. If T is greater than a7, then y = (T - a7)/(T + a6). If T is less than or = a7, then y = 0.
Enthalpy Function:
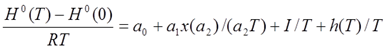 , with
, with
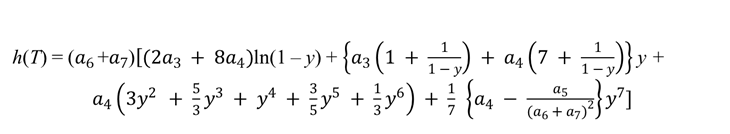
Entropy (at standard pressure po = 100 kPa):
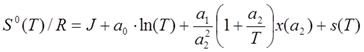 , with
, with
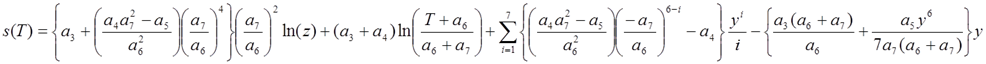 ,
,
where 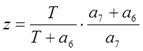 and
and 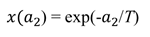
Equation Parameters: (Output details)
See Thermodynamics of Organic Compounds in the Gas State (Volumes I and II) by M. Frenkel, G. J. Kabo, K. N. Marsh, G. N. Roganov, and R. C. Wilhoit. Published by the Thermodynamics Research Center (TRC), College Station: TX. 1994. ISBN 1-883400-04-X